Introduction to Hydrolysis and Dehydration Reactions
Hydrolysis vs dehydration reactions are two of the most important chemical processes found in both nature and industry. Whether you’re digesting food, forming new molecules, or studying organic chemistry, these reactions quietly power major transformations. So what exactly do they mean, and how do they differ?
Why These Reactions Matter
You encounter these reactions every day sometimes without knowing it. From breaking down carbohydrates to forming proteins, hydrolysis and dehydration reactions are fundamental to life.
Everyday Examples Around You
Boiling pasta uses hydrolysis. Making plastic involves dehydration. Even your body relies on these reactions just to keep you alive.
What Is Hydrolysis?
Definition of Hydrolysis
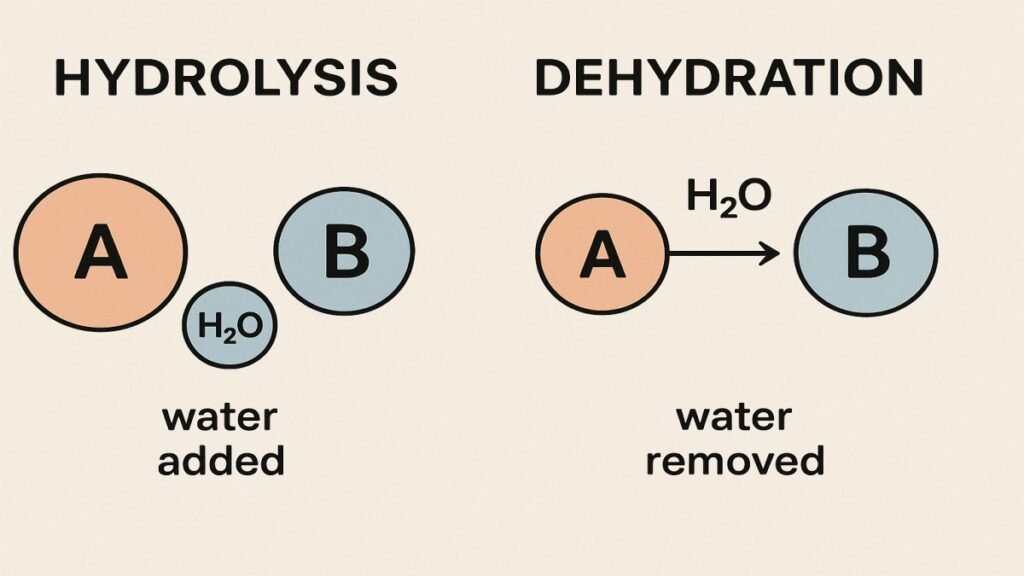
Hydrolysis is a chemical reaction where a molecule is split into two parts by adding water. The word literally means “water breaking.”
How Hydrolysis Works
In hydrolysis, a water molecule (H₂O) breaks apart into H⁺ and OH⁻. These ions attach to the fragments of the original molecule, causing it to split. Think of it as water acting like scissors.
Common Types of Hydrolysis
Acid Hydrolysis
Uses acids to speed up the reaction—common in digestion.
Base Hydrolysis
Uses bases and is often found in soap making and chemical processing.
Enzymatic Hydrolysis
Enzymes such as amylase or lipase help break down carbohydrates and fats.
Real-Life Examples of Hydrolysis
- Breakdown of starch into glucose in your mouth
- Digestion of fats in the small intestine
- Hydrolysis of esters to form alcohols and acids
What Is Dehydration (Condensation) Reaction?
Definition of Dehydration Reaction
A dehydration reaction happens when two molecules combine and release a water molecule. Also known as condensation, it forms larger, more complex structures.
How Dehydration Works
Two molecules react, lose a hydrogen (H) and hydroxyl group (OH), and form water. What remains joins together to create a new bond.
Major Types of Dehydration Reactions
Dehydration Synthesis
This is how your body forms proteins, DNA, and carbohydrates. Small units (monomers) join to make larger molecules (polymers).
Dehydration in Organic Chemistry
Alcohols often undergo dehydration to form alkenes. This is widely used in industrial chemistry.
Real-Life Examples of Dehydration Reactions
- Formation of proteins from amino acids
- Joining glucose molecules to form starch
- Manufacturing of plastics and synthetic fibers
Key Differences Between Hydrolysis and Dehydration
Water’s Role in Each Reaction
- Hydrolysis uses water to break molecules.
- Dehydration releases water to build molecules.
Energy Requirements
Hydrolysis often releases energy.
Dehydration typically requires energy input.
Biological Importance
Hydrolysis helps with digestion and energy release.
Dehydration helps build cell structures and genetic material.
Chemical Outcomes
Hydrolysis → smaller molecules
Dehydration → larger molecules
Hydrolysis vs Dehydration: Comparison Table
| Feature | Hydrolysis | Dehydration |
|---|---|---|
| Water involvement | Water added | Water removed |
| Result | Breakdown of molecules | Formation of larger molecules |
| Energy | Often releases energy | Requires energy |
| Example | Digestion of proteins | Formation of starch |
| Occurrence | Catabolic reaction | Anabolic reaction |
Why These Reactions Are Important in Biology
Digestion and Metabolism
Your body breaks down food using hydrolysis. Without it, you couldn’t absorb nutrients.
Building and Breaking Macromolecules
DNA, proteins, and carbohydrates all rely on dehydration to form—and hydrolysis to break apart.
Applications in Industry and Chemistry
Food Industry
Hydrolysis helps make syrups, softens foods, and enhances flavors.
Pharmaceutical Industry
Drug synthesis and breakdown often use dehydration and hydrolysis.
Polymer Manufacturing
Plastics, resins, and synthetic fibers depend heavily on dehydration reactions.
How to Easily Remember the Difference
Here’s a simple trick:
Hydrolysis = hydration = adding water.
Dehydration = drying = removing water.
If water goes in → hydrolysis.
If water comes out → dehydration.
Conclusion
Hydrolysis and dehydration reactions are powerful processes that shape biology, chemistry, and industry. Hydrolysis breaks molecules apart using water, while dehydration builds larger structures by removing water. Understanding these reactions helps you grasp everything from digestion to manufacturing. The more you explore them, the more you realize how essential they are to life and technology.
FAQs
1. Are hydrolysis and dehydration opposite reactions?
Yes. Hydrolysis breaks molecules apart, dehydration builds them up.
2. Why is hydrolysis important in digestion?
It breaks large molecules into absorbable units.
3. What type of reaction forms proteins?
Dehydration synthesis joins amino acids together.
4. Is dehydration the same as condensation?
Yes, both refer to removing water to form a new bond.
5. Can hydrolysis happen without enzymes?
Yes, but enzymes make it much faster.